
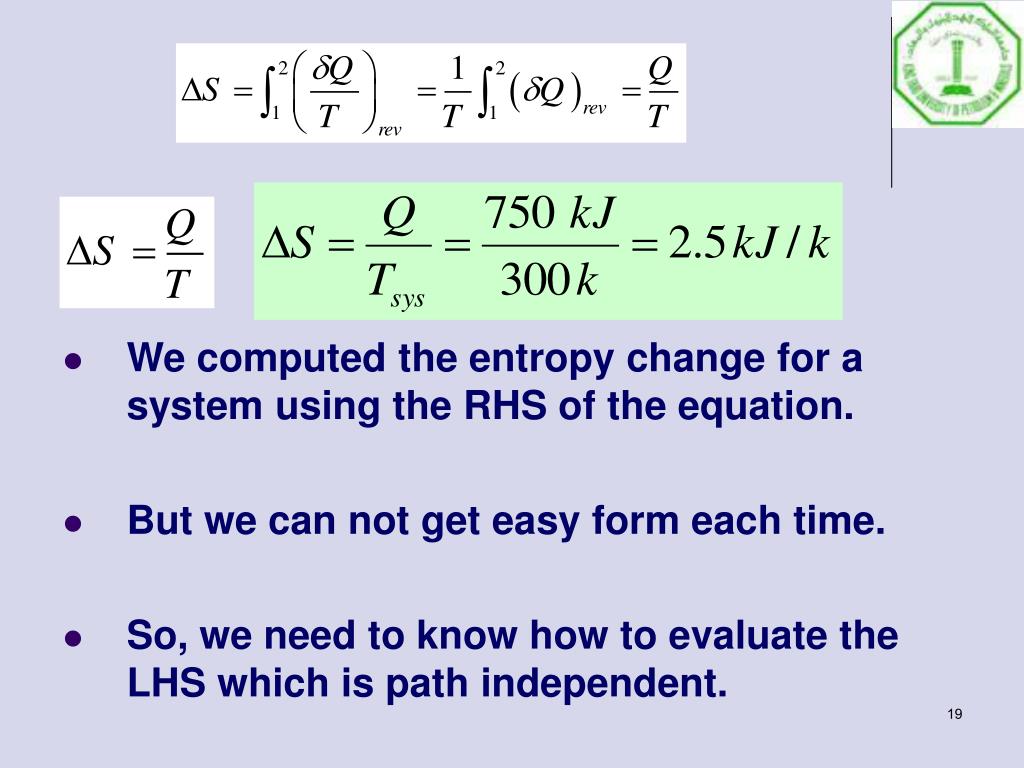
The fact that the absolute value of specific entropy is unknown is not a problem because it is the change in specific entropy (Δs) and not the absolute value that is important in practical problems. For example, the specific entropy of water or steam is given using the reference that the specific entropy of water is zero at 32☏. Also, like enthalpy, the entropy of a substance is given with respect to some reference value. Like enthalpy, entropy cannot be measured directly.
Entropy is represented by the letter S and can be defined as ΔS in the following relationships. Entropy is sometimes referred to as a measure of the inability to do work for a given heat transferred. Because entropy tells so much about the usefulness of an amount of heat transferred in performing work, the steam tables include values of specific entropy (s = S/m) as part of the information tabulated. Entropy quantifies the energy of a substance that is no longer available to perform useful work.

Because entropy is a property, changes in it can be determined by knowing the initial and final conditions of a substance. > Thermodynamics Directory | Heat Transfer DirectoryĮntropy Definition - Thermodynamic PropertiesĮntropy (S) is a property of a substance, as are pressure, temperature, volume, and enthalpy. As a result, a reversible process can change direction at any time, whereas an irreversible process cannot.Entropy Definition and Equation Thermodynamics In Section 4 we start our comparisons by considering strong. In contrast, an irreversible process is one in which the intermediate states are not equilibrium states, so change occurs spontaneously in only one direction. We then derive a new conservative formulation in terms of the entropy equation in Section 3. In a reversible process, every intermediate state between the extremes is an equilibrium state, regardless of the direction of the change.
Entropy equation how to#
Before discussing how to do so, however, we must understand the difference between a reversible process and an irreversible one. Based on the greater freedom of motion available to atoms in a liquid, we predict that the liquid sample will have the higher entropy.Ĭhanges in entropy (ΔS), together with changes in enthalpy (ΔH), enable us to predict in which direction a chemical or physical change will occur spontaneously. The nature of the atomic species is the same in both cases, but the phase is different: one sample is a solid, and one is a liquid.Hence we predict that the NH 3 sample will have the higher entropy. With four atoms instead of one, the NH 3 molecules have more motions available, leading to a greater number of microstates. Both substances are gases at 25☌, but one consists of He atoms and the other consists of NH 3 molecules.Given: amounts of substances and temperatureįrom the number of atoms present and the phase of each substance, predict which has the greater number of available microstates and hence the higher entropy. 1 mol of Pb(s) at 25☌ or 1 mol of Pb(l) at 800☌.1 mol of NH 3(g) or 1 mol of He(g), both at 25☌.Predict which substance in each pair has the higher entropy and justify your answer. The magnitude of the increase is greater than the magnitude of the decrease, so the overall entropy change for the formation of an NaCl solution is positive. Each hydrated ion, however, forms an ordered arrangement with water molecules, which decreases the entropy of the system. \( \newcommand\): The Effect of Solution Formation on Entropyĭissolving NaCl in water results in an increase in the entropy of the system.


 0 kommentar(er)
0 kommentar(er)
